If there is one thing that is worth a mention with deep sense of appreciation with pride, it is the role of Indian Pharmaceutical Manufactures during the time of crisis. Keeping the context and narrative limited to Indian scenario while the country fights the second wave of deadly pandemic, there are some bright spots to highlight. India was caught with a huge surprise in its management of COVID-19, with the second wave completely straining the country’s Healthcare Infrastructure. Along with the Healthcare Infrastructure limitations, it is the lack of appropriate medications used for emergency management that hurt the most. Initially it was the shortages with drugs like Remdesivir and Tocilizumab that impacted the management of emergency conditions. Now it is all about Amphotericin-B. In both scenarios, the Pharmaceutical Manufacturing capacity is being tested and the outcome is not a bad news for sure.
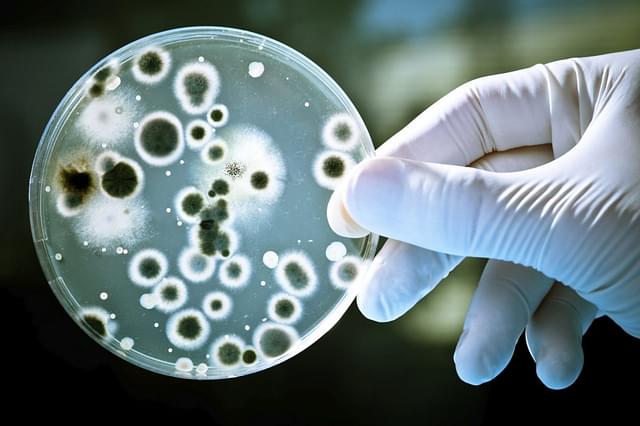
Mucormycosis or Black Fungus infections and the relevance of Amphotericin-B
According to the CDC official website, Mucormycosis is a rare fungal infection that affects the people with poor or weak immunity, attributed to their deteriorating health conditions. It is caused by the molds, technically known as mucormycetes, present in the external environment. It is not a new disease condition and the appropriate medication is already available with enough data and evidence to back its efficacy. But, the challenge in India has been about the availability of the drug to cater to the kind of demand the country had never seen in the past.
Now, the questions about the circumstances that led to such an increased demand only point towards the treatment protocols used at different levels in managing COVID. It was reported by multiple media outlets that the experts are of the opinion that the disproportionate use of steroids combined with the lack of medical hygiene while administering oxygen to the affected patients has caused this upsurge. The infected group of patients are identified as those patients who got treated for COVID with steroids with risk factors such as diabetes. The unexpected surge in the demand has only tested the ability of Indian Pharma to scale up its capacity and thankfully they seem to be approaching it the right way.
Indian Manufactures and Marketers credited for Amphotericin-B access
In the months of April and May when India first encountered the surge in Mucormycosis cases, there were five manufactures producing Amphotericin-B. They are Bharat Serums & Vaccines, BDR Pharmaceuticals, Sun Pharma, Cipla and Life Care Innovations. Mylan Labs has been importing this drug. These companies are likely to ramp up their production by around 60% by the month of June. This is certainly a good sign, considering the growing awareness levels among the medical professionals and general public about this disease.
Five more companies are granted the necessary approvals which will further scale up the production capacity. Emcure Pharmaceuticals, Natco Pharma, Gufic Biosciences, Alembic Pharmaceuticals and Lyca Pharmaceuticals are the companies with new manufacturing approvals. This certainly indicates the readiness of Indian Pharmaceutical Manufactures to conveniently adapt and exponentially improve their capacities when it comes to crisis situations and health emergencies. This can be further strengthened by the experiences they gather from this ongoing pandemic. The future is bright and promising.